Haematology Watch, Vol.7, Issue 1.
LABORATORY HAEMATOLOGY
Should we re-run an out-of-control Internal Quality Control result?
Dr. Mehmood ul Hasan Malik
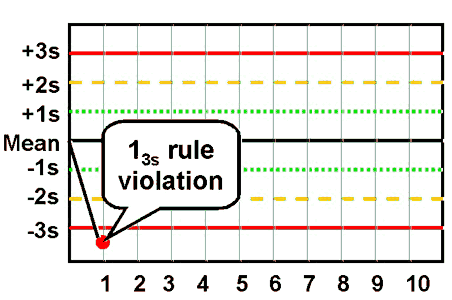
What should we do after getting an out-of-control result of our Internal Quality Control (IQC) such as the one given in the image* above?
A repeat?
A big NO....
Why not to repeat at first place?
This is due to the following:
1. suppose the IQC vial is expired, do you think that the repetition will change anything?
2. suppose that the IQC vial was not brought to room temperature, do you think that the repetition will change anything?
3. suppose that the IQC vial was not mixed, do you think that the repetition without mixing will change anything?
4. suppose that the IQC vial used was not the vial scanned, do you think that the repetition will change anything?
5. suppose that the IQC vial data was not entered correctly, do you think that the repetition will change anything?
6. suppose that the IQC vial was low in volume, do you think that the repetition will change anything?
7. suppose that the IQC vial lot written on the vial didn’t match with the data entered, do you think that the repetition will change anything?
8. suppose that the IQC vial level was not matching with the scanned data, do you think that the repetition will change anything?
9. suppose that the calibration was out, do you think that the repetition will change anything?
10. suppose that the reagent was low, do you think that the repetition will change anything?
11. suppose that a reagent of an analyte A was installed at the station of analyte B, do you think that the repetition will change anything?
12. what if PPM of the analyser was pending? do you think that the repetition will change anything?
Why people just repeat?
This is due to many perceptions:
1. People think that the goal is to maintain a flawless IQC record: this helps them in passing the visits.
2. Investigating an abnormal IQC result is problematic, so why to take pain?
3. Some people assume that just a repetition may remove the error behind the scene!
What if a repeated attempt benefits?
Well, even if a second result is acceptable, that doesn't mean that the error has gone! The error may persist but due to its intermittent nature, it has not interfered this second attempt e.g. an air bubble, which is commonly thought as the most likely random error. Even a random error like an air bubble needs identification & correction, not running away from it. Does a repetition remove the air bubble from the system?
Actually a repeat is like a "Try Try Again" event....
What is hidden behind the tip of this iceberg?
1. Firstly, the most serious disadvantage of repetition is that it shows that the laboratorian does not have any trust in his/her watch dogs!
2. Secondly, it shows that the laboratorian only gives importance to a normal result and is not willing to get any information from an unacceptable result!
3. Thirdly, it depicts that the Statistical Quality Control Procedure is only linked to normal results of IQC!
4. An IQC vial is a precious material which is not available to all the laboratories over the globe. And even those who get it are not in a surplus supply. So, as the number of errors increase, so does the usage of repeat IQC: assess it in your lab and forecast the wasted IQC vials for the 'repeat repeat" strategy!
What to do instead of repeating?
A good strategy is to avoid wasting precious IQC material, and JUST STOP TO INVESTIGATE the possible reason of out of control result.
THINK: WHY IT HAPPENED IN FIRST PLACE?
Finding the cause of alarm is as important as getting alarmed by using IQC!
Eliminate that reason, and only then re-run the IQC to see if the corrective action worked.
*The image belongs to: Westgard Rules | Multirules by James Westgard - Westgard QC
Good luck...
Designed by http://www.mitchinson.net